-

Our Mission
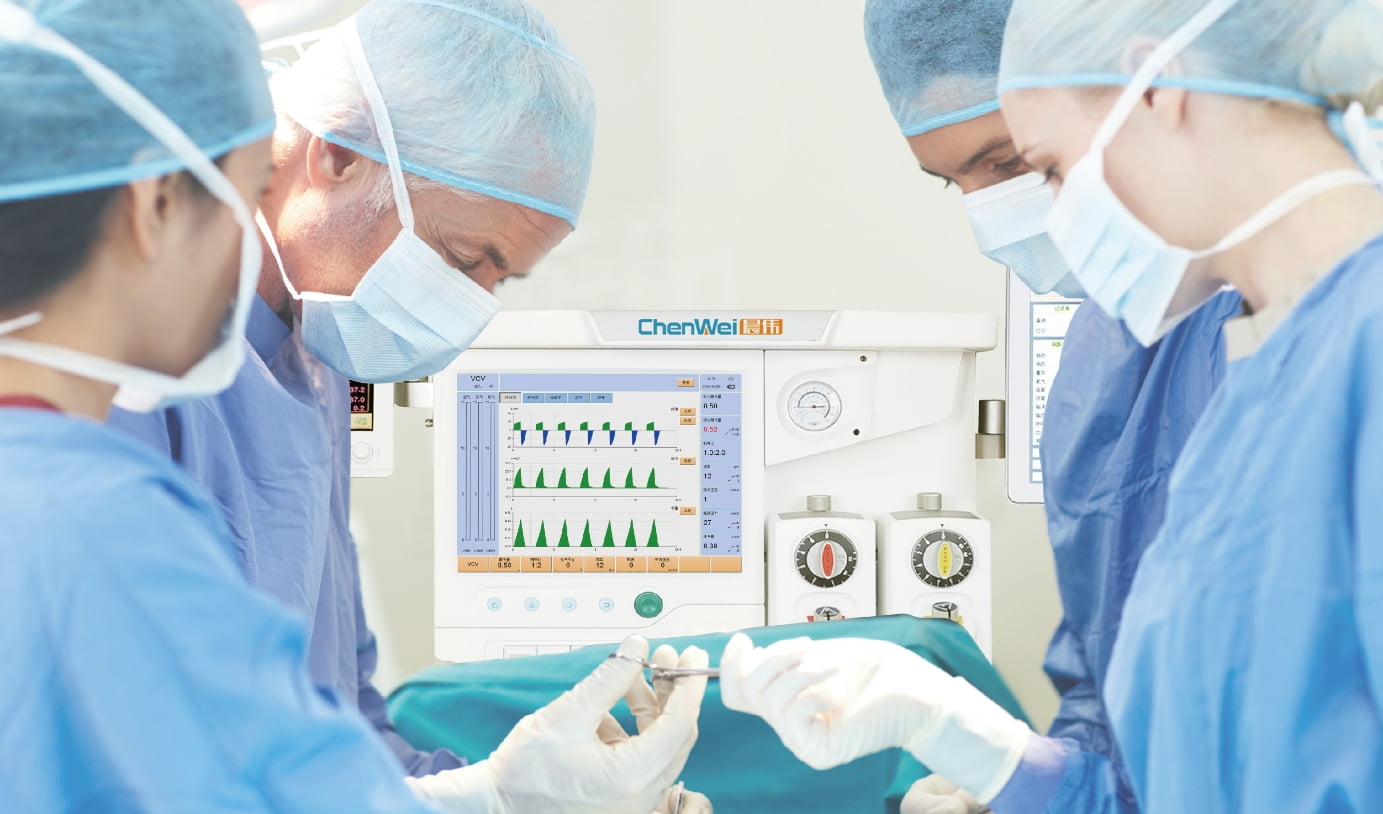
Care For Every Vital Moment -

Our Vision
Every critical moment of life support,
CHENWEI Stands by -

Core Values
Safe | Accurate | Efficient | Reliable




-
Ji NingxiangChairman and General Manager
Group Founder · Chief Technical Mentor · Pioneer of Anesthesia Technology Innovation in ChinaAs the founder and core leader of Chenwei Medical Group, Mr. Ji Ningxiang is a visionary who has deeply embedded technological innovation into the company's development. Serving concurrently as Chairman and General Manager, he has consistently anchored the company's growth in technological R&D while prioritizing clinical needs, guiding Chenwei Medical to continuously scale new heights in domestic high-end medical equipment technology.
With four decades of experience, Mr. Ji Ningxiang has remained at the forefront of medical technology R&D. His career epitomizes the evolution of anesthesia machine innovation in China:
He spearheaded the design of China's first digital display anesthesia machine, ushering in a new era of transition from analog needle gauges to digital displays and enhancing the precision and safety of anesthesia management.
He successfully developed China's first anesthesia machine equipped with a servo control system, achieving high-precision, intelligent control of ventilator gases—a major breakthrough in domestic technology for high-end performance.
These milestone achievements stem from his relentless pursuit and profound understanding of technological innovation. To date, Mr. Ji Ningxiang personally holds over ten independent intellectual property rights, including invention patents and utility model patents. These scientific accomplishments not only establish core technological barriers for Chenwei Medical but also propel technological advancement across the entire industry.As the company's Chief Technology Officer, he continues to personally guide R&D strategy, infusing his extensive experience and forward-thinking vision into every new product development. From frontline engineer to corporate leader, he remains steadfast in his belief that “technology is the lifeline of medical equipment.”
Under Mr. Ji Ningxiang's leadership, Chenwei Medical has evolved from a startup to a mature enterprise. Upholding the philosophy of “independent R&D and lean manufacturing,” the company is committed to providing reliable, advanced, and intelligent medical device solutions for the global market.
-
Ji NinglongExecutive Director and Deputy General Manager
Co-founder of Chenwei Medical GroupAs one of the core founders of Chenwei Medical Group, Mr. Ji Ninglong currently serves as Executive Director and Deputy General Manager, overseeing the formulation and implementation of the Group's domestic marketing strategy. Leveraging his extensive industry experience and keen market insight, he has successfully established a marketing network spanning East China, South China, North China, and Southwest China, while coordinating the daily operations of 26 provincial and municipal branches and offices nationwide.
Under Mr. Ji's leadership, Chenwei Medical's domestic marketing team has continuously optimized channel distribution and enhanced customer service, achieving steady business expansion and significantly boosting brand influence. With over three decades of dedication to the healthcare industry, he has consistently adhered to a market-driven, customer-centric approach, establishing Chenwei Medical's outstanding brand image and industry reputation in the Chinese market.
During the COVID-19 pandemic, Mr. Ji stepped up to lead the group's coordinated response, swiftly mobilizing resources to support frontline medical workers with robust material supplies and technical assistance. His exceptional leadership and outstanding contributions earned national recognition, leading his team to receive the State Council's “National Advanced Collective in Pandemic Response” honorary medal.
His strategic planning and execution capabilities, coupled with his sense of mission during major societal events, have laid a solid foundation for Chenwei Medical's deepening market presence and expansion within China. He remains a vital driving force behind the Group's sustained innovation and development.
-
Ji YeExecutive Director and Vice President in charge of overseas operations
Ms. Ji Ye holds a Master of Science degree in Communication Engineering from the University of Manchester, UK. She is fully responsible for Chenwei Medical's global market channel development, strategic planning formulation, operational management system optimization, and brand internationalization, dedicated to enhancing the Group's comprehensive competitiveness in international markets. Throughout her career, she managed Chenwei Medical's collaboration with the U.S. Space Lab medical project as the OEM program lead, demonstrating extensive experience in international project coordination and cross-disciplinary collaboration alongside exceptional technical comprehension and resource integration capabilities. She also spearheaded overseas market development for flagship products, overseeing requirements analysis, functional design, multilingual version development, and testing/release.
During the COVID-19 global pandemic, Ms. Ji Ye proactively fulfilled corporate social responsibility by urgently activating a global response mechanism. She led the international business team in fully supporting pandemic relief efforts across multiple countries, personally coordinating closely with numerous ambassadors and institutions in China. She efficiently organized the global allocation and delivery of pandemic supplies, providing critical support to the international community's fight against the virus. This earned recognition from multiple governments, significantly enhancing Chenwei Medical's brand reputation and international influence.
With over fifteen years of focus on overseas marketing, Ms. Ji Ye possesses profound insights and localized practical experience in global policy environments, market structures, and customer needs. She has successfully driven the implementation of multiple strategic projects, demonstrating exceptional regional market development and high-level negotiation capabilities. Simultaneously, she places high importance on talent strategy, actively recruiting and cultivating high-caliber overseas talent to build a high-performing, diverse international team. This has laid a solid talent and organizational foundation for the sustainable development of Chenwei Medical's global operations. Leveraging her deep industry expertise, international management perspective, and exceptional leadership, Ms. Ji Ye continues to propel Chenwei Medical toward a more prominent position in the global healthcare market.
-
Ji ShuweiOverseas Department Marketing Director and Management Representative
Mr. Ji Shuwei holds a Master's degree in International Marketing from Newcastle University. He possesses extensive experience in market research and development across Europe, Africa, and Asia. He is highly proficient in ventilator and anesthesia system products. Currently, he is primarily responsible for overseas market expansion and promotion, strategic planning, and product development. As a medical technology expert deeply versed in global market strategies, he serves as the Overseas Marketing Director at Chenwei Medical. With his forward-thinking market insights and exceptional leadership, he continuously advances the branding and globalization of China's high-end medical equipment on the international stage.
Mr. Ji Shuwei holds a Master of Science in International Marketing from Newcastle University, UK, establishing a robust theoretical foundation and international perspective. After graduation, he focused on the medical equipment sector, accumulating deep expertise in the highly technical ventilator and anesthesia systems product lines. He stands as one of the industry's rare hybrid talents combining market development capabilities with profound product knowledge. His career has been dedicated to expanding into overseas territories, with valuable experience in developing diverse markets across Europe, Africa, and Asia. This expertise is reflected not only in successful market entry strategies but also in his profound understanding and flexible adaptation to regional cultural differences, regulatory policies, and channel characteristics. He excels at conducting precise market research to identify unmet clinical needs and market opportunities, then executing strategic plans based on these insights.
Currently serving as the strategic leader for overseas markets, he oversees the localization and compliance implementation across international regions while guiding market development throughout new product cycles. His mission is to connect Chenwei Medical's innovative products with the health needs of patients worldwide, continuously driving robust growth for the company's global operations.
ensuring safer, more reliable medical support to equally safeguard every critical moment of life.

- Heilongjiang
- Nei Mongol
- Liaoning
- Xinjiang
- Hebei
- Shandong
- Shanxi
- Gansu
- Shanxi
- Henan
- Yunnan
- Hubei
- Anhui
- Zhejiang
- Hunan
- Jiangxi
- Guangxi
- Fujian
- Guangdong
-
Research StrengthsOver three decades of dedicated effort have equipped Chenwei Medical with extensive production expertise. Concurrently, our professional R&D team ensures we possess not only advanced manufacturing capabilities but also substantial innovation capacity.
-
Equipment AdvantagesWe have introduced internationally leading fully automated production lines and precision testing equipment to ensure every process adheres to a high-standard quality control system, achieving efficient, precise, and stable equipment manufacturing.
-
Production AdvantagesThrough intelligent manufacturing systems and rigorous process management, each piece of equipment achieves full process control, significantly enhancing production efficiency and product consistency and meeting the market demand for high-quality medical devices.
-
Material AdvantagesWe meticulously select stable, medical-grade raw materials and implement a rigorous supplier screening process to ensure product safety and reliability from the source.

We channel over 20% of our annual revenue into R&D, empowering a dedicated team of 100+ experts. Their work is validated by a growing portfolio of novel patents and 100+ software copyrights.
-
100+ItemPatents Filed
-
100+ItemSoftware Copyrights
-
36+ItemAwards & Honors